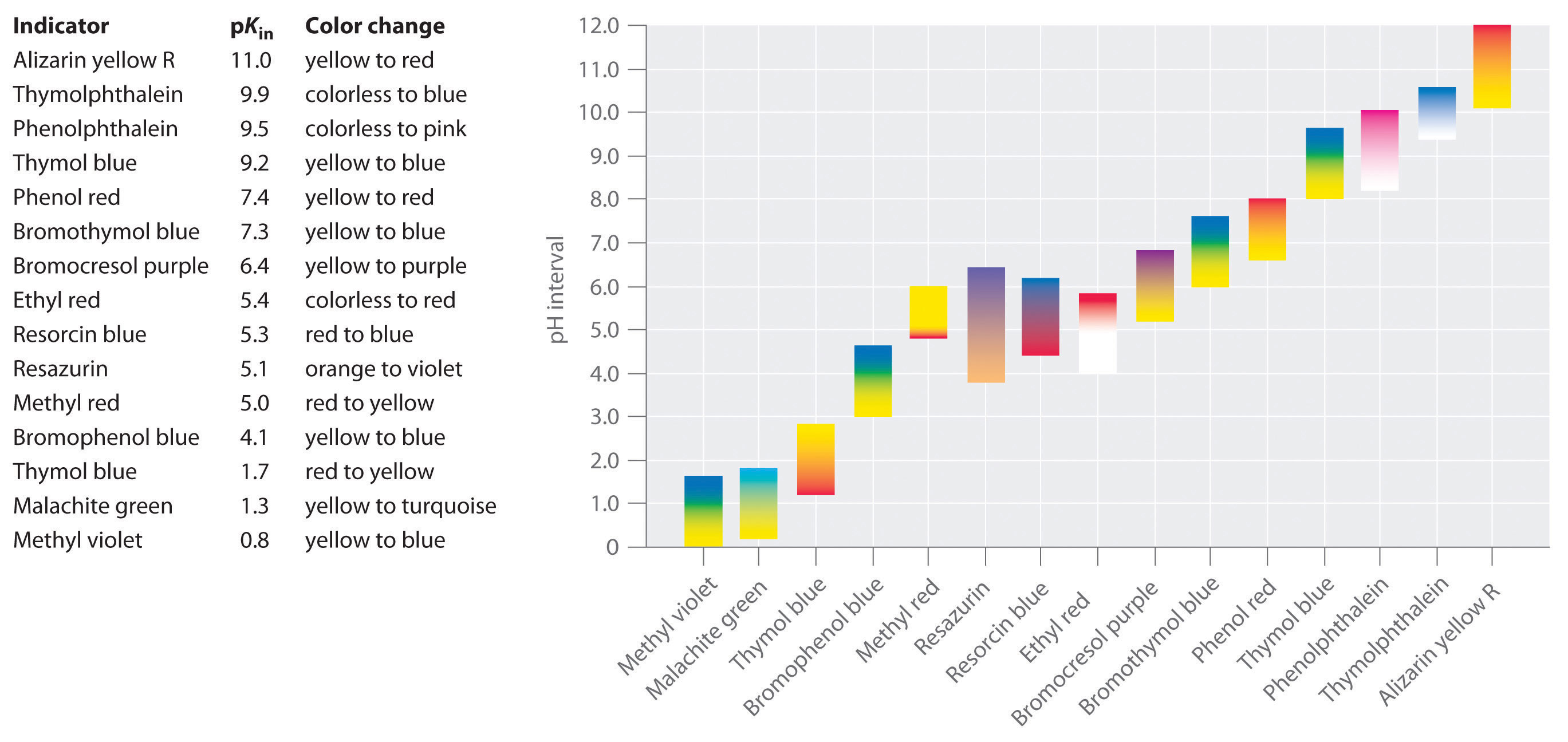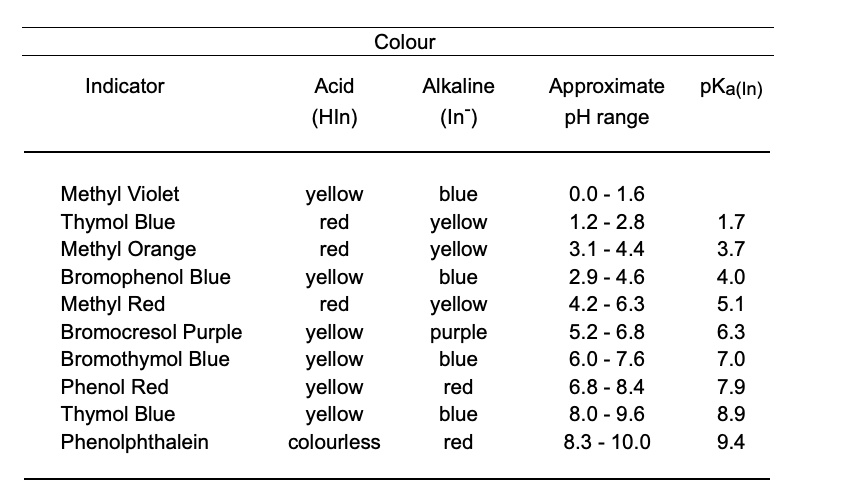
SOLVED:Colour Indicator Acid (HIn) Alkaline (In" ) Approximate pH range pKa(ln) Methyl Violet Thymol Blue Methyl Orange Bromophenol Blue Methyl Red Bromocresol Purple Bromothymol Blue Phenol Red Thymol Blue Phenolphthalein yellow red

VOLUMETRIC ANALYSIS Volumetric analysis is an analysis in which the amount of the unknown is calculated from a known volume of added solution. - ppt video online download

Effect of pH and HCl Concentration on the Distribution of Methyl Orange... | Download Scientific Diagram

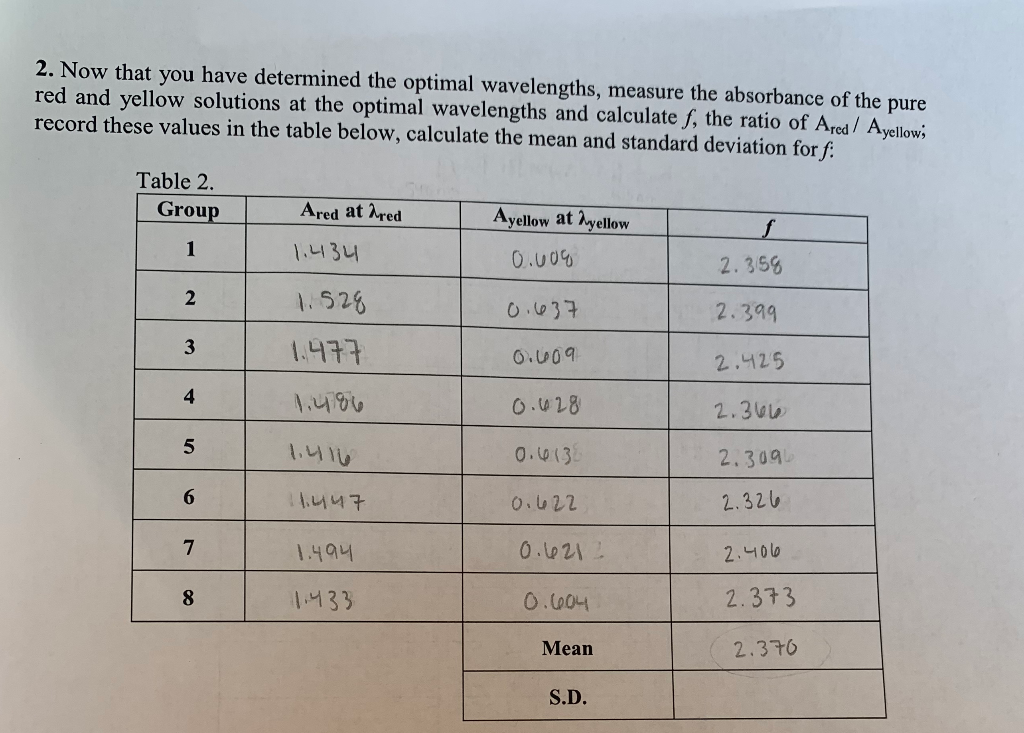
Determination of the Ka of methyl Red Indicator by Colorimetry - Spectroscopy applications: Determination of the Ka of Methyl Red Indicator by | Course Hero
![Ionisation of water and pH For any Bronsted conjugate Acid-Base pair pH concept pH = -log[H + ] pX = -logX pH scale [H + ] > M, pH < 7 ACIDIC [H. - ppt download Ionisation of water and pH For any Bronsted conjugate Acid-Base pair pH concept pH = -log[H + ] pX = -logX pH scale [H + ] > M, pH < 7 ACIDIC [H. - ppt download](https://images.slideplayer.com/26/8569566/slides/slide_13.jpg)
Ionisation of water and pH For any Bronsted conjugate Acid-Base pair pH concept pH = -log[H + ] pX = -logX pH scale [H + ] > M, pH < 7 ACIDIC [H. - ppt download
![PDF) [Chem 28] Spectrophotometric Determination of theAcid Dissociation Constant of Methyl Red | Eliora Maris Medrano - Academia.edu PDF) [Chem 28] Spectrophotometric Determination of theAcid Dissociation Constant of Methyl Red | Eliora Maris Medrano - Academia.edu](https://0.academia-photos.com/attachment_thumbnails/37647595/mini_magick20190301-20875-kmph4s.png?1551429838)
PDF) [Chem 28] Spectrophotometric Determination of theAcid Dissociation Constant of Methyl Red | Eliora Maris Medrano - Academia.edu